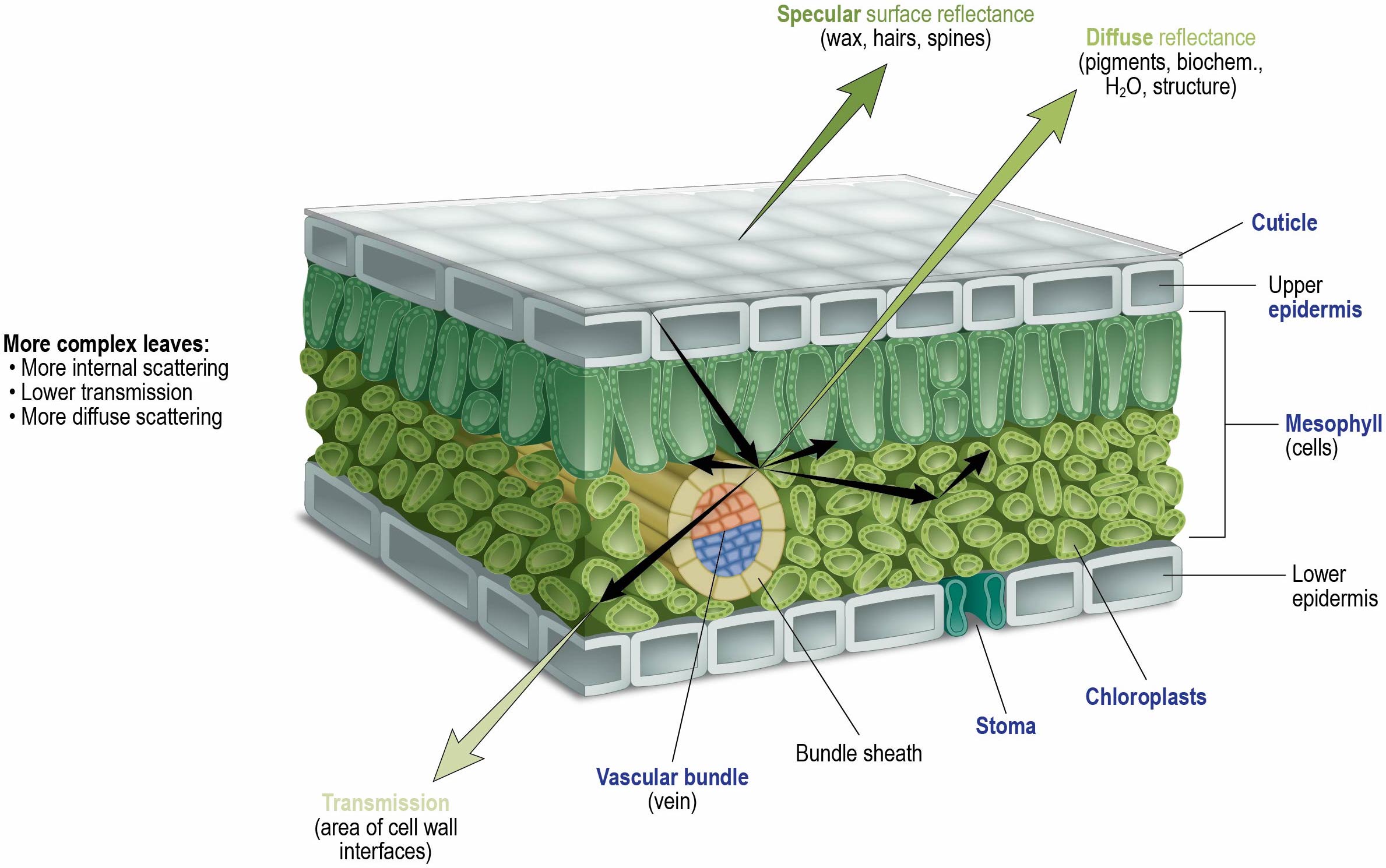
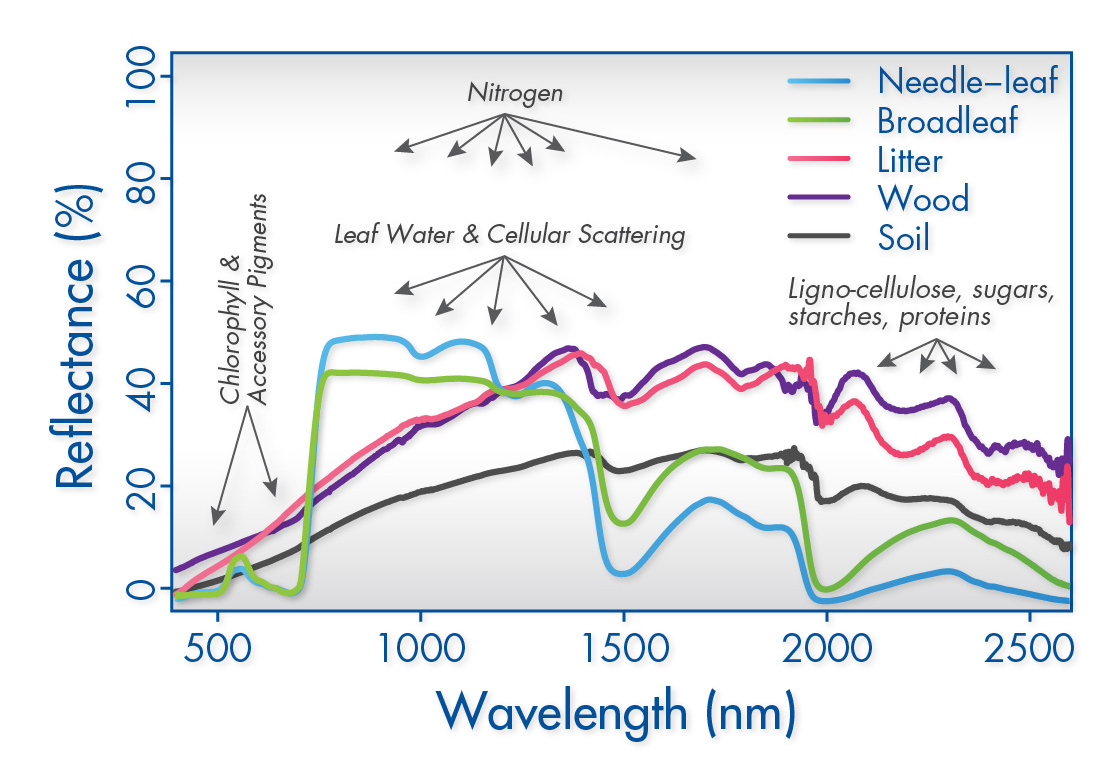
-
Notifications
You must be signed in to change notification settings - Fork 9
Example Tutorial #02: Ely et al. 2019 dataset
Shawn P. Serbin edited this page Jun 19, 2024
·
1 revision
Spectra-trait PLSR example using leaf-level spectra and leaf nitrogen content (Narea, g/m2) data from eight different crop species growing in a glasshouse at Brookhaven National Laboratory. This example illustrates running the PLSR permutation by group
Shawn P. Serbin, Julien Lamour, & Jeremiah Anderson 2024-06-19
This is an R Markdown Notebook to illustrate how to load an internal dataset (“ely_plsr_data”), choose the “optimal” number of plsr components, and fit a plsr model for leaf nitrogen content (Narea, g/m2)
DOI: https://doi.org/10.1093/jxb/erz061
list.of.packages <- c("pls","dplyr","here","plotrix","ggplot2","gridExtra","spectratrait")
invisible(lapply(list.of.packages, library, character.only = TRUE))## Warning: package 'pls' was built under R version 4.3.1
##
## Attaching package: 'pls'
## The following object is masked from 'package:stats':
##
## loadings
## Warning: package 'dplyr' was built under R version 4.3.1
##
## Attaching package: 'dplyr'
## The following objects are masked from 'package:stats':
##
## filter, lag
## The following objects are masked from 'package:base':
##
## intersect, setdiff, setequal, union
## here() starts at /Users/sserbin/Library/CloudStorage/OneDrive-NASA/Data/Github/spectratrait
## Warning: package 'plotrix' was built under R version 4.3.1
## Warning: package 'ggplot2' was built under R version 4.3.1
##
## Attaching package: 'gridExtra'
## The following object is masked from 'package:dplyr':
##
## combine
### Setup options
# Script options
pls::pls.options(plsralg = "oscorespls")
pls::pls.options("plsralg")## $plsralg
## [1] "oscorespls"
# Default par options
opar <- par(no.readonly = T)
# Specify output directory, output_dir
# Options:
# tempdir - use a OS-specified temporary directory
# user defined PATH - e.g. "~/scratch/PLSR"
output_dir <- "tempdir"data("ely_plsr_data")
head(ely_plsr_data)[,1:8]## Species_Code Common_Name C_N_mass C_g_m2 H20_g_m2 LMA_g_m2 N_g_m2
## 1 HEAN3 common sunflower 7.58 15.61210 167.63 36.40 2.103694
## 2 HEAN3 common sunflower 8.33 14.73724 164.68 34.65 1.231713
## 3 HEAN3 common sunflower 7.70 15.02495 156.95 35.08 1.764752
## 4 CUSA4 garden cucumber 7.40 11.14835 111.52 26.23 1.287963
## 5 CUSA4 garden cucumber 7.47 11.60735 123.58 26.71 1.411361
## 6 CUSA4 garden cucumber 7.43 8.06035 114.36 18.40 1.117704
## Wave_500
## 1 4.782000
## 2 4.341714
## 3 4.502857
## 4 3.333429
## 5 3.313571
## 6 3.272286
# What is the target variable?
inVar <- "N_g_m2"## [1] "/private/var/folders/th/fpt_z3417gn8xgply92pvy6r0000gq/T/RtmpMXBwDv"
Start.wave <- 500
End.wave <- 2400
wv <- seq(Start.wave,End.wave,1)
plsr_data <- ely_plsr_data
head(plsr_data)[,1:6]## Species_Code Common_Name C_N_mass C_g_m2 H20_g_m2 LMA_g_m2
## 1 HEAN3 common sunflower 7.58 15.61210 167.63 36.40
## 2 HEAN3 common sunflower 8.33 14.73724 164.68 34.65
## 3 HEAN3 common sunflower 7.70 15.02495 156.95 35.08
## 4 CUSA4 garden cucumber 7.40 11.14835 111.52 26.23
## 5 CUSA4 garden cucumber 7.47 11.60735 123.58 26.71
## 6 CUSA4 garden cucumber 7.43 8.06035 114.36 18.40
### Create cal/val datasets
## Make a stratified random sampling in the strata USDA_Species_Code and Domain
method <- "base" #base/dplyr
# base R - a bit slow
# dplyr - much faster
split_data <- spectratrait::create_data_split(dataset=plsr_data, approach=method,
split_seed=23452135, prop=0.7,
group_variables="Species_Code")## HEAN3 Cal: 70%
## CUSA4 Cal: 68.182%
## CUPE Cal: 70.588%
## SOLYL Cal: 70%
## OCBA Cal: 68.421%
## POPUL Cal: 71.429%
## GLMA4 Cal: 70.588%
## PHVU Cal: 66.667%
names(split_data)## [1] "cal_data" "val_data"
cal.plsr.data <- split_data$cal_data
head(cal.plsr.data)[1:8]## Species_Code Common_Name C_N_mass C_g_m2 H20_g_m2 LMA_g_m2 N_g_m2
## 1 HEAN3 common sunflower 7.58 15.61210 167.63 36.40 2.103694
## 2 HEAN3 common sunflower 8.33 14.73724 164.68 34.65 1.231713
## 4 CUSA4 garden cucumber 7.40 11.14835 111.52 26.23 1.287963
## 6 CUSA4 garden cucumber 7.43 8.06035 114.36 18.40 1.117704
## 7 CUPE field pumpkin 7.20 11.43007 128.42 25.83 1.215333
## 10 SOLYL garden tomato 7.89 11.61918 142.23 27.40 1.304110
## Wave_500
## 1 4.782000
## 2 4.341714
## 4 3.333429
## 6 3.272286
## 7 2.943143
## 10 4.145714
val.plsr.data <- split_data$val_data
head(val.plsr.data)[1:8]## Species_Code Common_Name C_N_mass C_g_m2 H20_g_m2 LMA_g_m2 N_g_m2
## 3 HEAN3 common sunflower 7.70 15.024947 156.95 35.08 1.7647515
## 5 CUSA4 garden cucumber 7.47 11.607347 123.58 26.71 1.4113615
## 8 CUPE field pumpkin 7.67 12.466238 124.67 29.22 1.1468413
## 9 CUPE field pumpkin 7.64 17.100448 142.85 43.39 1.1390174
## 13 SOLYL garden tomato 7.73 7.938866 129.95 17.96 0.9483533
## 15 OCBA sweet basil 8.13 16.975969 173.30 38.65 1.1246459
## Wave_500
## 3 4.502857
## 5 3.313571
## 8 2.868000
## 9 3.338286
## 13 3.960286
## 15 3.744000
rm(split_data)
# Datasets:
print(paste("Cal observations: ",dim(cal.plsr.data)[1],sep=""))## [1] "Cal observations: 124"
print(paste("Val observations: ",dim(val.plsr.data)[1],sep=""))## [1] "Val observations: 54"
cal_hist_plot <- ggplot(data = cal.plsr.data,
aes(x = cal.plsr.data[,paste0(inVar)])) +
geom_histogram(fill=I("grey50"),col=I("black"),alpha=I(.7)) +
labs(title=paste0("Calibration Histogram for ",inVar), x = paste0(inVar),
y = "Count")
val_hist_plot <- ggplot(data = val.plsr.data,
aes(x = val.plsr.data[,paste0(inVar)])) +
geom_histogram(fill=I("grey50"),col=I("black"),alpha=I(.7)) +
labs(title=paste0("Validation Histogram for ",inVar), x = paste0(inVar),
y = "Count")
histograms <- grid.arrange(cal_hist_plot, val_hist_plot, ncol=2)## `stat_bin()` using `bins = 30`. Pick better value with `binwidth`.
## `stat_bin()` using `bins = 30`. Pick better value with `binwidth`.

ggsave(filename = file.path(outdir,paste0(inVar,"_Cal_Val_Histograms.png")),
plot = histograms,
device="png", width = 30,
height = 12, units = "cm",
dpi = 300)
# output cal/val data
write.csv(cal.plsr.data,file=file.path(outdir,paste0(inVar,'_Cal_PLSR_Dataset.csv')),
row.names=FALSE)
write.csv(val.plsr.data,file=file.path(outdir,paste0(inVar,'_Val_PLSR_Dataset.csv')),
row.names=FALSE)### Format PLSR data for model fitting
cal_spec <- as.matrix(cal.plsr.data[, which(names(cal.plsr.data) %in% paste0("Wave_",wv))])
cal.plsr.data <- data.frame(cal.plsr.data[, which(names(cal.plsr.data) %notin% paste0("Wave_",wv))],
Spectra=I(cal_spec))
head(cal.plsr.data)[1:5]## Species_Code Common_Name C_N_mass C_g_m2 H20_g_m2
## 1 HEAN3 common sunflower 7.58 15.61210 167.63
## 2 HEAN3 common sunflower 8.33 14.73724 164.68
## 4 CUSA4 garden cucumber 7.40 11.14835 111.52
## 6 CUSA4 garden cucumber 7.43 8.06035 114.36
## 7 CUPE field pumpkin 7.20 11.43007 128.42
## 10 SOLYL garden tomato 7.89 11.61918 142.23
val_spec <- as.matrix(val.plsr.data[, which(names(val.plsr.data) %in% paste0("Wave_",wv))])
val.plsr.data <- data.frame(val.plsr.data[, which(names(val.plsr.data) %notin% paste0("Wave_",wv))],
Spectra=I(val_spec))
head(val.plsr.data)[1:5]## Species_Code Common_Name C_N_mass C_g_m2 H20_g_m2
## 3 HEAN3 common sunflower 7.70 15.024947 156.95
## 5 CUSA4 garden cucumber 7.47 11.607347 123.58
## 8 CUPE field pumpkin 7.67 12.466238 124.67
## 9 CUPE field pumpkin 7.64 17.100448 142.85
## 13 SOLYL garden tomato 7.73 7.938866 129.95
## 15 OCBA sweet basil 8.13 16.975969 173.30
par(mfrow=c(1,2)) # B, L, T, R
spectratrait::f.plot.spec(Z=cal.plsr.data$Spectra,wv=wv,plot_label="Calibration")
spectratrait::f.plot.spec(Z=val.plsr.data$Spectra,wv=wv,plot_label="Validation")
dev.copy(png,file.path(outdir,paste0(inVar,'_Cal_Val_Spectra.png')),
height=2500,width=4900, res=340)## quartz_off_screen
## 3
dev.off();## quartz_off_screen
## 2
par(mfrow=c(1,1))### Use permutation to determine the optimal number of components
if(grepl("Windows", sessionInfo()$running)){
pls.options(parallel = NULL)
} else {
pls.options(parallel = parallel::detectCores()-1)
}
method <- "firstMin" #firstPlateau, firstMin
random_seed <- 1245565
seg <- 50
maxComps <- 16
iterations <- 80
prop <- 0.70
nComps <- spectratrait::find_optimal_comp_by_groups(dataset=cal.plsr.data, targetVariable=inVar,
method=method, maxComps=maxComps,
iterations=iterations, prop=prop,
random_seed=random_seed,
group_variables="Species_Code")## [1] "*** Identifying optimal number of PLSR components using stratified resampling by group_variables ***"
## [1] "*** Running permutation test. Please hang tight, this can take awhile ***"
## [1] "Options:"
## [1] "Max Components: 16 Iterations: 80 Data Proportion (percent): 70"
## [1] "*** Providing PRESS and coefficient array output ***"
## No id variables; using all as measure variables
## [1] "*** Optimal number of components based on t.test: 15"

dev.copy(png,file.path(outdir,paste0(paste0(inVar,"_PLSR_Component_Selection.png"))),
height=2800, width=3400, res=340)## quartz_off_screen
## 3
dev.off();## quartz_off_screen
## 2
plsr.out <- plsr(as.formula(paste(inVar,"~","Spectra")),scale=FALSE,ncomp=nComps,validation="LOO",
trace=FALSE,data=cal.plsr.data)
fit <- plsr.out$fitted.values[,1,nComps]
pls.options(parallel = NULL)
# External validation fit stats
par(mfrow=c(1,2)) # B, L, T, R
pls::RMSEP(plsr.out, newdata = val.plsr.data)## (Intercept) 1 comps 2 comps 3 comps 4 comps 5 comps
## 0.5908 0.4735 0.4162 0.4037 0.3347 0.3023
## 6 comps 7 comps 8 comps 9 comps 10 comps 11 comps
## 0.2993 0.3081 0.2814 0.2445 0.2276 0.2104
## 12 comps 13 comps 14 comps 15 comps
## 0.1954 0.2003 0.1973 0.2108
plot(pls::RMSEP(plsr.out,estimate=c("test"),newdata = val.plsr.data), main="MODEL RMSEP",
xlab="Number of Components",ylab="Model Validation RMSEP",lty=1,col="black",cex=1.5,lwd=2)
box(lwd=2.2)
pls::R2(plsr.out, newdata = val.plsr.data)## (Intercept) 1 comps 2 comps 3 comps 4 comps 5 comps
## -0.004079 0.355010 0.501632 0.531088 0.677620 0.737143
## 6 comps 7 comps 8 comps 9 comps 10 comps 11 comps
## 0.742224 0.726835 0.772115 0.827942 0.850962 0.872685
## 12 comps 13 comps 14 comps 15 comps
## 0.890124 0.884529 0.887961 0.872129
plot(pls::R2(plsr.out,estimate=c("test"),newdata = val.plsr.data), main="MODEL R2",
xlab="Number of Components",ylab="Model Validation R2",lty=1,col="black",cex=1.5,lwd=2)
box(lwd=2.2)
dev.copy(png,file.path(outdir,paste0(paste0(inVar,"_Validation_RMSEP_R2_by_Component.png"))),
height=2800, width=4800, res=340)## quartz_off_screen
## 3
dev.off();## quartz_off_screen
## 2
par(opar)#calibration
cal.plsr.output <- data.frame(cal.plsr.data[, which(names(cal.plsr.data) %notin% "Spectra")],
PLSR_Predicted=fit,
PLSR_CV_Predicted=as.vector(plsr.out$validation$pred[,,nComps]))
cal.plsr.output <- cal.plsr.output %>%
mutate(PLSR_CV_Residuals = PLSR_CV_Predicted-get(inVar))
head(cal.plsr.output)## Species_Code Common_Name C_N_mass C_g_m2 H20_g_m2 LMA_g_m2 N_g_m2
## 1 HEAN3 common sunflower 7.58 15.61210 167.63 36.40 2.103694
## 2 HEAN3 common sunflower 8.33 14.73724 164.68 34.65 1.231713
## 4 CUSA4 garden cucumber 7.40 11.14835 111.52 26.23 1.287963
## 6 CUSA4 garden cucumber 7.43 8.06035 114.36 18.40 1.117704
## 7 CUPE field pumpkin 7.20 11.43007 128.42 25.83 1.215333
## 10 SOLYL garden tomato 7.89 11.61918 142.23 27.40 1.304110
## PLSR_Predicted PLSR_CV_Predicted PLSR_CV_Residuals
## 1 1.836047 1.714086 -0.38960842
## 2 1.530813 1.685388 0.45367526
## 4 1.254794 1.262835 -0.02512724
## 6 1.127053 1.129340 0.01163542
## 7 1.196259 1.188471 -0.02686200
## 10 1.276380 1.281683 -0.02242624
cal.R2 <- round(pls::R2(plsr.out,intercept=F)[[1]][nComps],2)
cal.RMSEP <- round(sqrt(mean(cal.plsr.output$PLSR_CV_Residuals^2)),2)
val.plsr.output <- data.frame(val.plsr.data[, which(names(val.plsr.data) %notin% "Spectra")],
PLSR_Predicted=as.vector(predict(plsr.out,
newdata = val.plsr.data,
ncomp=nComps, type="response")[,,1]))
val.plsr.output <- val.plsr.output %>%
mutate(PLSR_Residuals = PLSR_Predicted-get(inVar))
head(val.plsr.output)## Species_Code Common_Name C_N_mass C_g_m2 H20_g_m2 LMA_g_m2 N_g_m2
## 3 HEAN3 common sunflower 7.70 15.024947 156.95 35.08 1.7647515
## 5 CUSA4 garden cucumber 7.47 11.607347 123.58 26.71 1.4113615
## 8 CUPE field pumpkin 7.67 12.466238 124.67 29.22 1.1468413
## 9 CUPE field pumpkin 7.64 17.100448 142.85 43.39 1.1390174
## 13 SOLYL garden tomato 7.73 7.938866 129.95 17.96 0.9483533
## 15 OCBA sweet basil 8.13 16.975969 173.30 38.65 1.1246459
## PLSR_Predicted PLSR_Residuals
## 3 1.7624701 -0.002281391
## 5 1.2947218 -0.116639722
## 8 0.9934199 -0.153421396
## 9 1.1345273 -0.004490078
## 13 0.7432855 -0.205067758
## 15 1.1613789 0.036733007
val.R2 <- round(pls::R2(plsr.out,newdata=val.plsr.data,intercept=F)[[1]][nComps],2)
val.RMSEP <- round(sqrt(mean(val.plsr.output$PLSR_Residuals^2)),2)
rng_quant <- quantile(cal.plsr.output[,inVar], probs = c(0.001, 0.999))
cal_scatter_plot <- ggplot(cal.plsr.output, aes(x=PLSR_CV_Predicted, y=get(inVar))) +
theme_bw() + geom_point() + geom_abline(intercept = 0, slope = 1, color="dark grey",
linetype="dashed", linewidth=1.5) +
xlim(rng_quant[1], rng_quant[2]) +
ylim(rng_quant[1], rng_quant[2]) +
labs(x=paste0("Predicted ", paste(inVar), " (units)"),
y=paste0("Observed ", paste(inVar), " (units)"),
title=paste0("Calibration: ", paste0("Rsq = ", cal.R2), "; ", paste0("RMSEP = ",
cal.RMSEP))) +
theme(axis.text=element_text(size=18), legend.position="none",
axis.title=element_text(size=20, face="bold"),
axis.text.x = element_text(angle = 0,vjust = 0.5),
panel.border = element_rect(linetype = "solid", fill = NA, linewidth=1.5))
cal_resid_histogram <- ggplot(cal.plsr.output, aes(x=PLSR_CV_Residuals)) +
geom_histogram(alpha=.5, position="identity") +
geom_vline(xintercept = 0, color="black",
linetype="dashed", linewidth=1) + theme_bw() +
theme(axis.text=element_text(size=18), legend.position="none",
axis.title=element_text(size=20, face="bold"),
axis.text.x = element_text(angle = 0,vjust = 0.5),
panel.border = element_rect(linetype = "solid", fill = NA, linewidth=1.5))
rng_quant <- quantile(val.plsr.output[,inVar], probs = c(0.001, 0.999))
val_scatter_plot <- ggplot(val.plsr.output, aes(x=PLSR_Predicted, y=get(inVar))) +
theme_bw() + geom_point() + geom_abline(intercept = 0, slope = 1, color="dark grey",
linetype="dashed", linewidth=1.5) +
xlim(rng_quant[1], rng_quant[2]) +
ylim(rng_quant[1], rng_quant[2]) +
labs(x=paste0("Predicted ", paste(inVar), " (units)"),
y=paste0("Observed ", paste(inVar), " (units)"),
title=paste0("Validation: ", paste0("Rsq = ", val.R2), "; ", paste0("RMSEP = ",
val.RMSEP))) +
theme(axis.text=element_text(size=18), legend.position="none",
axis.title=element_text(size=20, face="bold"),
axis.text.x = element_text(angle = 0,vjust = 0.5),
panel.border = element_rect(linetype = "solid", fill = NA, linewidth=1.5))
val_resid_histogram <- ggplot(val.plsr.output, aes(x=PLSR_Residuals)) +
geom_histogram(alpha=.5, position="identity") +
geom_vline(xintercept = 0, color="black",
linetype="dashed", linewidth=1) + theme_bw() +
theme(axis.text=element_text(size=18), legend.position="none",
axis.title=element_text(size=20, face="bold"),
axis.text.x = element_text(angle = 0,vjust = 0.5),
panel.border = element_rect(linetype = "solid", fill = NA, linewidth=1.5))
# plot cal/val side-by-side
scatterplots <- grid.arrange(cal_scatter_plot, val_scatter_plot, cal_resid_histogram,
val_resid_histogram, nrow=2,ncol=2)## Warning: Removed 5 rows containing missing values or values outside the scale range
## (`geom_point()`).
## Warning: Removed 4 rows containing missing values or values outside the scale range
## (`geom_point()`).
## `stat_bin()` using `bins = 30`. Pick better value with `binwidth`.
## `stat_bin()` using `bins = 30`. Pick better value with `binwidth`.
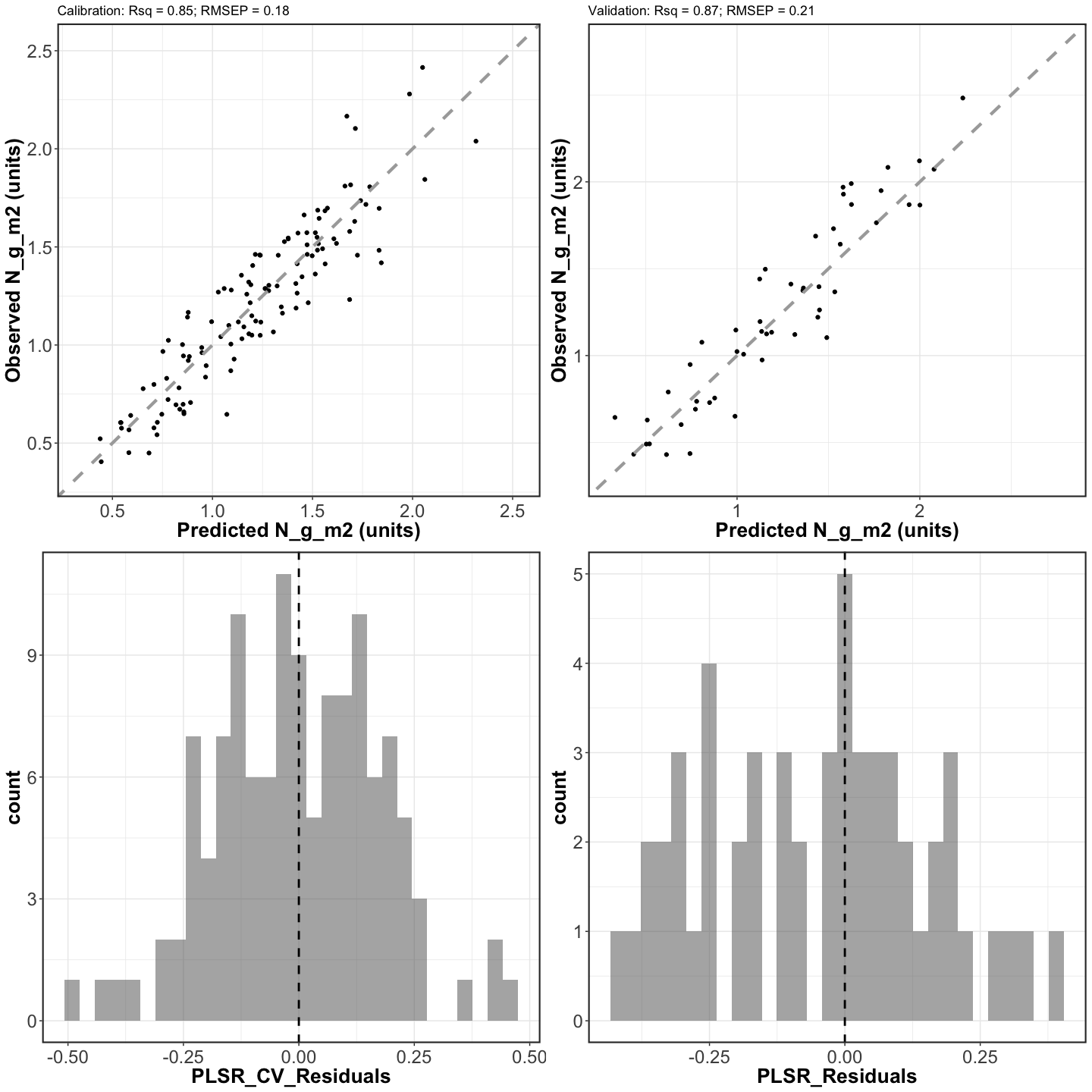
ggsave(filename = file.path(outdir,paste0(inVar,"_Cal_Val_Scatterplots.png")),
plot = scatterplots, device="png",
width = 32,
height = 30, units = "cm",
dpi = 300)vips <- spectratrait::VIP(plsr.out)[nComps,]
par(mfrow=c(2,1))
plot(plsr.out, plottype = "coef",xlab="Wavelength (nm)",
ylab="Regression coefficients",legendpos = "bottomright",
ncomp=nComps,lwd=2)
box(lwd=2.2)
plot(seq(Start.wave,End.wave,1),vips,xlab="Wavelength (nm)",ylab="VIP",cex=0.01)
lines(seq(Start.wave,End.wave,1),vips,lwd=3)
abline(h=0.8,lty=2,col="dark grey")
box(lwd=2.2)
dev.copy(png,file.path(outdir,paste0(inVar,'_Coefficient_VIP_plot.png')),
height=3100, width=4100, res=340)## quartz_off_screen
## 3
dev.off();## quartz_off_screen
## 2
if(grepl("Windows", sessionInfo()$running)){
pls.options(parallel =NULL)
} else {
pls.options(parallel = parallel::detectCores()-1)
}
### PLSR bootstrap permutation uncertainty analysis
iterations <- 500 # how many permutation iterations to run
prop <- 0.70 # fraction of training data to keep for each iteration
plsr_permutation <- spectratrait::pls_permutation_by_groups(dataset=cal.plsr.data,
targetVariable=inVar,
maxComps=nComps,
iterations=iterations,
prop=prop, group_variables="Species_Code",
verbose=FALSE)## [1] "*** Running permutation test. Please hang tight, this can take awhile ***"
## [1] "Options:"
## [1] "Max Components: 15 Iterations: 500 Data Proportion (percent): 70"
## [1] "*** Providing PRESS and coefficient array output ***"
bootstrap_intercept <- plsr_permutation$coef_array[1,,nComps]
bootstrap_coef <- plsr_permutation$coef_array[2:length(plsr_permutation$coef_array[,1,nComps]),
,nComps]
rm(plsr_permutation)
# apply coefficients to left-out validation data
interval <- c(0.025,0.975)
Bootstrap_Pred <- val.plsr.data$Spectra %*% bootstrap_coef +
matrix(rep(bootstrap_intercept, length(val.plsr.data[,inVar])), byrow=TRUE,
ncol=length(bootstrap_intercept))
Interval_Conf <- apply(X = Bootstrap_Pred, MARGIN = 1, FUN = quantile,
probs=c(interval[1], interval[2]))
sd_mean <- apply(X = Bootstrap_Pred, MARGIN = 1, FUN = sd)
sd_res <- sd(val.plsr.output$PLSR_Residuals)
sd_tot <- sqrt(sd_mean^2+sd_res^2)
val.plsr.output$LCI <- Interval_Conf[1,]
val.plsr.output$UCI <- Interval_Conf[2,]
val.plsr.output$LPI <- val.plsr.output$PLSR_Predicted-1.96*sd_tot
val.plsr.output$UPI <- val.plsr.output$PLSR_Predicted+1.96*sd_tot
head(val.plsr.output)## Species_Code Common_Name C_N_mass C_g_m2 H20_g_m2 LMA_g_m2 N_g_m2
## 3 HEAN3 common sunflower 7.70 15.024947 156.95 35.08 1.7647515
## 5 CUSA4 garden cucumber 7.47 11.607347 123.58 26.71 1.4113615
## 8 CUPE field pumpkin 7.67 12.466238 124.67 29.22 1.1468413
## 9 CUPE field pumpkin 7.64 17.100448 142.85 43.39 1.1390174
## 13 SOLYL garden tomato 7.73 7.938866 129.95 17.96 0.9483533
## 15 OCBA sweet basil 8.13 16.975969 173.30 38.65 1.1246459
## PLSR_Predicted PLSR_Residuals LCI UCI LPI UPI
## 3 1.7624701 -0.002281391 1.5710330 1.9443661 1.3151243 2.209816
## 5 1.2947218 -0.116639722 1.2019841 1.4531979 0.8688563 1.720587
## 8 0.9934199 -0.153421396 0.8544582 1.1646561 0.5564158 1.430424
## 9 1.1345273 -0.004490078 0.9954061 1.2824287 0.7007745 1.568280
## 13 0.7432855 -0.205067758 0.5836738 0.9094675 0.3042086 1.182362
## 15 1.1613789 0.036733007 1.0021191 1.2849671 0.7291004 1.593657
# Bootstrap regression coefficient plot
spectratrait::f.plot.coef(Z = t(bootstrap_coef), wv = wv,
plot_label="Bootstrap regression coefficients",position = 'bottomleft')
abline(h=0,lty=2,col="grey50")
box(lwd=2.2)
dev.copy(png,file.path(outdir,paste0(inVar,'_Bootstrap_Regression_Coefficients.png')),
height=2100, width=3800, res=340)## quartz_off_screen
## 3
dev.off();## quartz_off_screen
## 2
rmsep_percrmsep <- spectratrait::percent_rmse(plsr_dataset = val.plsr.output,
inVar = inVar,
residuals = val.plsr.output$PLSR_Residuals,
range="full")
RMSEP <- rmsep_percrmsep$rmse
perc_RMSEP <- rmsep_percrmsep$perc_rmse
r2 <- round(pls::R2(plsr.out, newdata = val.plsr.data, intercept=F)$val[nComps],2)
expr <- vector("expression", 3)
expr[[1]] <- bquote(R^2==.(r2))
expr[[2]] <- bquote(RMSEP==.(round(RMSEP,2)))
expr[[3]] <- bquote("%RMSEP"==.(round(perc_RMSEP,2)))
rng_vals <- c(min(val.plsr.output$LPI), max(val.plsr.output$UPI))
par(mfrow=c(1,1), mar=c(4.2,5.3,1,0.4), oma=c(0, 0.1, 0, 0.2))
plotrix::plotCI(val.plsr.output$PLSR_Predicted,val.plsr.output[,inVar],
li=val.plsr.output$LPI, ui=val.plsr.output$UPI, gap=0.009,sfrac=0.000,
lwd=1.6, xlim=c(rng_vals[1], rng_vals[2]), ylim=c(rng_vals[1], rng_vals[2]),
err="x", pch=21, col="black", pt.bg=scales::alpha("grey70",0.7), scol="grey80",
cex=2, xlab=paste0("Predicted ", paste(inVar), " (units)"),
ylab=paste0("Observed ", paste(inVar), " (units)"),
cex.axis=1.5,cex.lab=1.8)
abline(0,1,lty=2,lw=2)
plotrix::plotCI(val.plsr.output$PLSR_Predicted,val.plsr.output[,inVar],
li=val.plsr.output$LCI, ui=val.plsr.output$UCI, gap=0.009,sfrac=0.004,
lwd=1.6, xlim=c(rng_vals[1], rng_vals[2]), ylim=c(rng_vals[1], rng_vals[2]),
err="x", pch=21, col="black", pt.bg=scales::alpha("grey70",0.7), scol="black",
cex=2, xlab=paste0("Predicted ", paste(inVar), " (units)"),
ylab=paste0("Observed ", paste(inVar), " (units)"),
cex.axis=1.5,cex.lab=1.8, add=T)
legend("topleft", legend=expr, bty="n", cex=1.5)
legend("bottomright", legend=c("Prediction Interval","Confidence Interval"),
lty=c(1,1), col = c("grey80","black"), lwd=3, bty="n", cex=1.5)
box(lwd=2.2)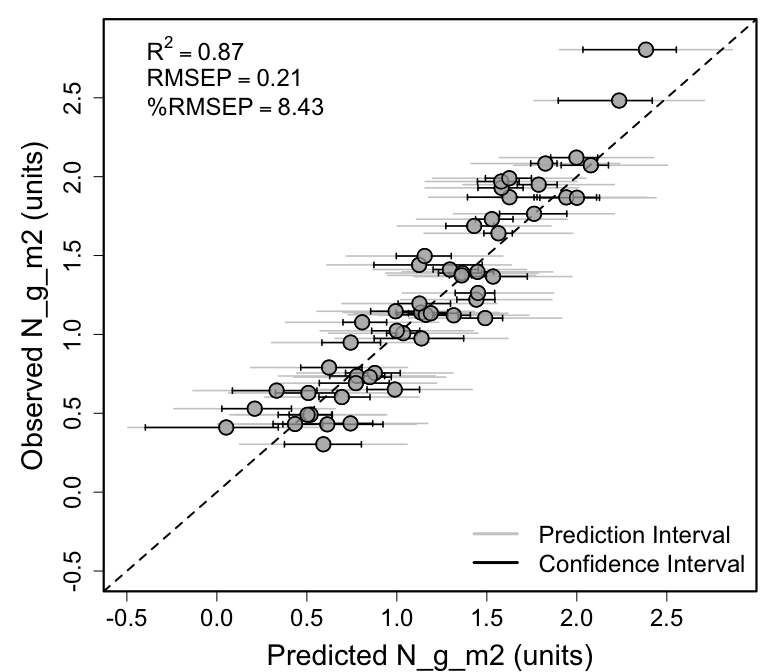
dev.copy(png,file.path(outdir,paste0(inVar,"_PLSR_Validation_Scatterplot.png")),
height=2800, width=3200, res=340)## quartz_off_screen
## 3
dev.off();## quartz_off_screen
## 2
# Bootstrap Coefficients
out.jk.coefs <- data.frame(Iteration=seq(1,length(bootstrap_intercept),1),
Intercept=bootstrap_intercept,t(bootstrap_coef))
names(out.jk.coefs) <- c("Iteration","Intercept",paste0("Wave_",wv))
head(out.jk.coefs)[1:6]## Iteration Intercept Wave_500 Wave_501 Wave_502 Wave_503
## 1 1 0.4731951 0.0236618987 0.021719096 0.023063691 0.02187741
## 2 2 0.5415203 -0.0007012397 0.001892634 0.008241293 0.01105366
## 3 3 0.6512533 0.0123054098 0.013428257 0.015824665 0.01772586
## 4 4 -0.9976728 0.0145306759 0.016119715 0.018834952 0.01959049
## 5 5 0.1267626 0.0076041315 0.007329090 0.009971693 0.01339406
## 6 6 0.8509641 0.0139793124 0.015195593 0.015170417 0.01434085
write.csv(out.jk.coefs,file=file.path(outdir,paste0(inVar,
'_Bootstrap_PLSR_Coefficients.csv')),
row.names=FALSE)print(paste("Output directory: ", outdir))## [1] "Output directory: /var/folders/th/fpt_z3417gn8xgply92pvy6r0000gq/T//RtmpMXBwDv"
# Observed versus predicted
write.csv(cal.plsr.output,file=file.path(outdir,
paste0(inVar,'_Observed_PLSR_CV_Pred_',
nComps,'comp.csv')),
row.names=FALSE)
# Validation data
write.csv(val.plsr.output,file=file.path(outdir,
paste0(inVar,'_Validation_PLSR_Pred_',
nComps,'comp.csv')),
row.names=FALSE)
# Model coefficients
coefs <- coef(plsr.out,ncomp=nComps,intercept=TRUE)
write.csv(coefs,file=file.path(outdir,
paste0(inVar,'_PLSR_Coefficients_',
nComps,'comp.csv')),
row.names=TRUE)
# PLSR VIP
write.csv(vips,file=file.path(outdir,
paste0(inVar,'_PLSR_VIPs_',
nComps,'comp.csv')))print("**** PLSR output files: ")## [1] "**** PLSR output files: "
print(list.files(outdir)[grep(pattern = inVar, list.files(outdir))])## [1] "N_g_m2_Bootstrap_PLSR_Coefficients.csv"
## [2] "N_g_m2_Bootstrap_Regression_Coefficients.png"
## [3] "N_g_m2_Cal_PLSR_Dataset.csv"
## [4] "N_g_m2_Cal_Val_Histograms.png"
## [5] "N_g_m2_Cal_Val_Scatterplots.png"
## [6] "N_g_m2_Cal_Val_Spectra.png"
## [7] "N_g_m2_Coefficient_VIP_plot.png"
## [8] "N_g_m2_Observed_PLSR_CV_Pred_15comp.csv"
## [9] "N_g_m2_PLSR_Coefficients_15comp.csv"
## [10] "N_g_m2_PLSR_Component_Selection.png"
## [11] "N_g_m2_PLSR_Validation_Scatterplot.png"
## [12] "N_g_m2_PLSR_VIPs_15comp.csv"
## [13] "N_g_m2_Val_PLSR_Dataset.csv"
## [14] "N_g_m2_Validation_PLSR_Pred_15comp.csv"
## [15] "N_g_m2_Validation_RMSEP_R2_by_Component.png"